In general when the fertigation is shifting from soil media to soilless media which is almost an inert one,(actually as hydroponics) the media has an almost zero nutrients retention capacity, a lack of buffer capacity, the requirements for both water and nutrients are more intensive, therefore the nutrient solution has to supply all plant nutrients accurately based on the required amount per each physiological stage of the plant, and unlike soil media any mistake might be resulted in crop damage.
Concepts & Definitions
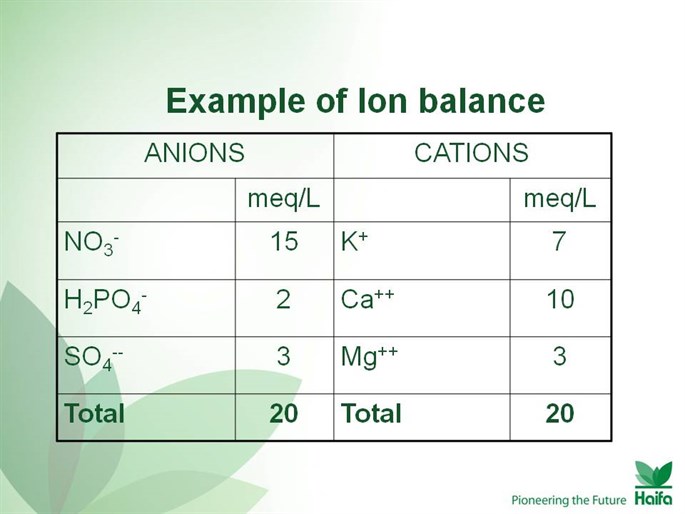
- When preparing the nutrient solution the principle of ionic equilibrium should be kept based on the sum of anions = sum of cations as long as their concentration in solution is expressed in meq/L. ( Milliequivalent/liter). The sum of 10 meq/L in the solution will supply a 1 unit of EC ( electrical conductivity ) of 1 dS/M ( deciSiemens/meter).
- Example for H2SO4: ( the weight of 1 mol has to divide by its valence in order to get the equivalent /liter value as in the case of Mg, Ca & S which are all bivalent ).
2H = 1 x 2 = 2
S= 32 x 1 = 32
0= 16 x 4 = 64
Total = 98 grams of sulfuric acid in one liter of solution = 1millimol/liter
98/ 2 = 49 grams of sulfuric acid In one liter of solution = 1 milliequivalent / liter.
- Conversion from meq/L to PPM = meq/L x atomic weight . for instance , 5 meq /L of K = 5 x 39 = 195 ppm of K .
Example of Ion Balance: