When preparing a fertilizer stock solution, there are several details that should be noted to get stability, avoid precipitations, and maintain the best nutritional values for the plants.
The tanks
The tanks should be of opaque material that does not transmit light, and well-sealed.
This is necessary to avoid dirt, development of biotic organisms, and UV damage to chelates.
Water source
Knowing the quality of your irrigation water allows you to create a better fertilization program while saving unnecessary costs. EC, pH, and bicarbonates are critical factors that you should consider.
It is advisable to get a lab analysis if lab services are available in your region.
- Alkalinity (high pH) is closely related to the presence of bicarbonates (HCO3-). High levels of bicarbonates increase the risk for precipitation in your tank.
- Add acids to counter bicarbonates and to adjust the pH. Added acids will first neutralize the bicarbonates, and only then they’ll lower the pH.
- Bicarbonates increase the electrical conductivity (EC) of the solution. EC can't be reduced by using acids.
- The acids add nutrients (nitrogen, phosphorus or sulfur) to the stock solution. Take this into account in your calculations.
Compatibility
The two -tank system enables separation of fertilizers that might form precipitates when dissolved together.
Note:
- calcium and magnesium fertilizers should be dissolved separately from phosphorus and sulfur fertilizers.
- In a single-tank system, make sure that the pH is between 3-4.
When magnesium sulfate is mixed in the same tank with phosphorus fertilizers, the pH of the solution must not exceed 5. Use the table to set fertilizer composition in each tank.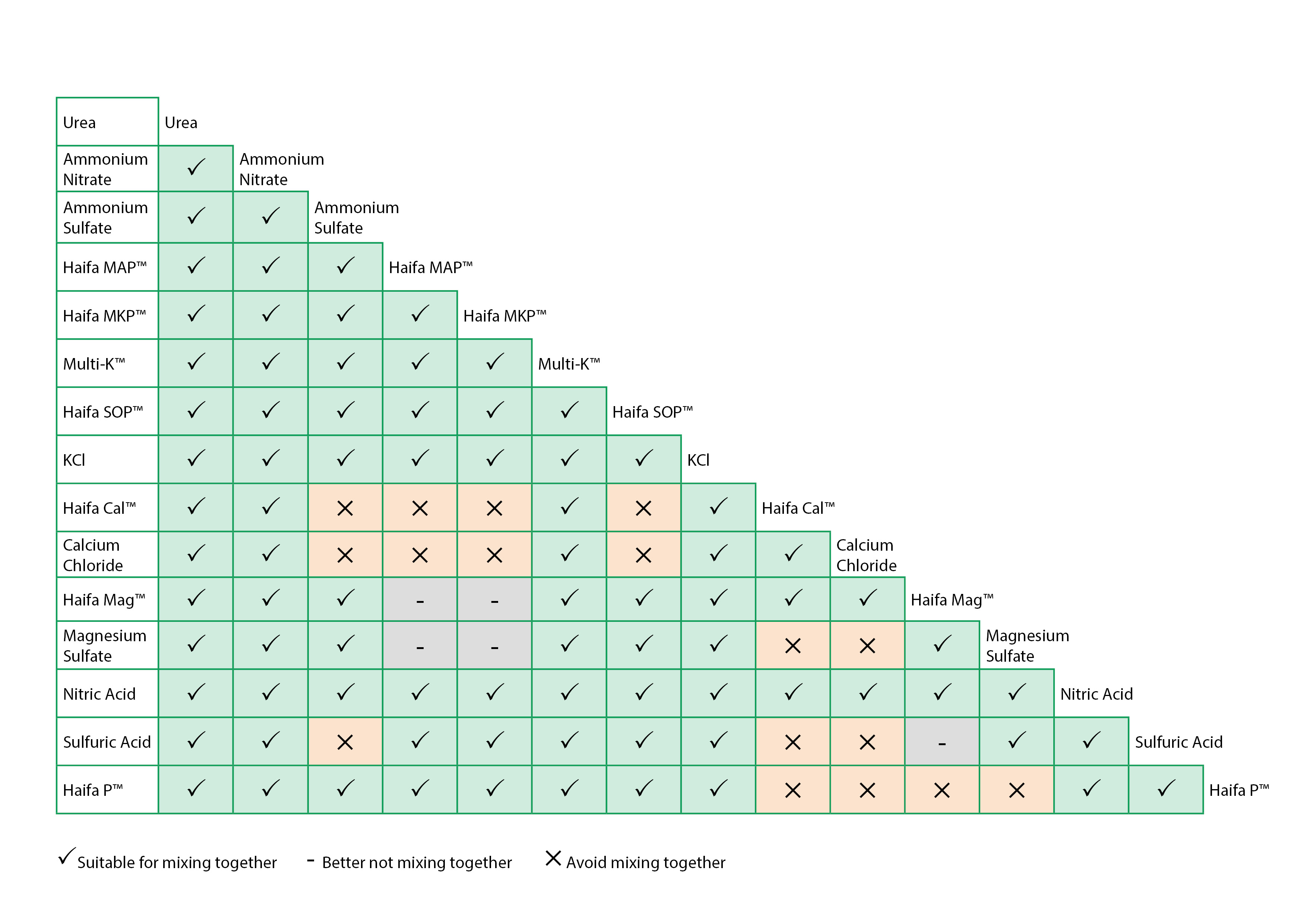
Micronutrients
Micronutrients can be added to the solution either as sulfur-based compounds or as chelates.
- In the form of chelates, micronutrients are protected from precipitation, thus more available for plant uptake. However, the stability of chelates depends on pH. When choosing chelates, the following must be considered:
- stock solution & drip water pH
- soil or substrate pH
- Water disinfection equipment (UV or acidification) might affect certain chelates
- Sulfur-based compounds are pH indifferent and cost less, but they form precipitates easily, thus becoming unavailable for plant uptake.
Preparing the fertilizer solution
Temperature: As solubility increases with temperature, higher water temperature enables higher concentration of nutrients in the stock solution.
Maximum recommended concentration is 20% (e.g., 200 kg fertilizers per m3)
At low water temperatures, use concentrations between 10-15%.
- Fill 1/4 - 1/3 of the spray tank with water.
- Add the fertilizer(s) gradually while filling the rest of the tank with water.
- Use an electric stirrer or an educator nozzle to improve dissolution rate.
Note: fertilizers of lower solubility will dissolve better if applied first. Follow the order advised below.
| TANK A (calcium and magnesium) | TANK B (phosphorus and sulfur) |
| Adjust the pH to 5-7. If acid is needed, use only nitric acid. Stir and wait for the solution to stabilize. | Set the pH level below 5, ideally around 4. Stir and wait for the solution to stabilize. Polyphosphate may be applied at this stage. If you use Haifa VitaPhos-K™, readjust pH. With Haifa GrowClean™, pH adjustment is unnecessary. |
| Advised order of fertilizer mixing into the solution | |
* MgNO3 is always preferred in tank A. It can be applied to tank B if pH is lower than 5. |
* Different SOP products affect the pH differently. Consider this when adjusting the pH. |
| Micronutrients | |
| |
Suitability of micronutrients in fertilizer solutions:
| pH stability range | Suitable for | Tank | Comments | |
| Sulphur based: MnSO4 ZnSO4 CuSO4 FeSO4 | pH Indifferent | Foliar application Acidic soils | A, B |
|
| Fe-EDTA | 1-6.5 | Foliar application Acidic soils | A |
|
| Fe-DTPA | 2-8 | Soilless greenhouses Recirculating systems | A |
|
| Fe-EDDHA | 4- 12 | Soilless greenhouses Recirculating systems Alkaline soils Calcareous soils | A |
|
| Fe-HBED | 4-12 | Soilless greenhouses Recirculating systems Alkaline soils Calcareous soils | A |
|
| FE-HEEDTA | 2-8 | UV disinfection systems Acidic soils Soilless greenhouses | A |
|
| Cu-EDTA Zn-EDTA | 1.5-10 | All soils & Soilless Greenhouses Open Field | A, B |
|
| Mn-EDTA | 3-10 | Soil & Soilless Greenhouses Open Field | A, B |
|
 |  |  |
| Read more about Greenhouse Fertilizers | Advanced Nutritional Solutions for Soilless Crops | Water Soluble Fertilizers |



