One of main function of the acids is to neutralize the bicarbonates
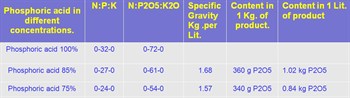
In the photo above: Example of different concentrations of phosphoric acid and its technical grades.
One of main function of the acids is to neutralize the bicarbonates – HCO3 in the water by mean that each miliequivalent of acid is neutralizing 1 meq. Of HCO3 thus reducing the risk of forming calcium carbonate precipitates (CaCO3 ).
This is occurring and relevant in hard water where the content of HCO3 is more than 150 ppm or 2.5 Meq. /Lit and usually with Ca ˃ 60 ppm ( 3 Meq. ), the Mg ˃ 30 ppm ( 2.5 Meq.), and the pH is alkaline ˃ 7.5
0.5 – 1 meq. Of HCO3 should be remained in the water in order to maintain its stability – buffer capacity.
Precipitaion of CaCO3 in the drip area due to not using acid in order to neutralizing HCO3:




