Index
- Leaf analysis
- Nitrogen (N)
- Phosphorous (P)
- Potassium (K)
- Calcium (Ca)
- Magnesium (Mg)
- Sulfur (S)
- Manganese (Mn)
- Iron (Fe)
- Boron (B)
- Zinc (Zn)
- Molybdenum (Mo)
- Copper (Cu)
3.1 Leaf analysis
Visual diagnosis of disorders can be confused by symptoms induced by non-nutritional factors such as disease, pests and chemicals. Leaf analysis can be used to confirm a visual diagnosis. This involves chemical testing of leaves to establish whether specific nutrients are present in plant tissue at normal concentrations. This technique can be used to check the suitability of a fertilizer program and to anticipate the need for nutrient supplements. A disadvantage of leaf analysis is that it is slow: most laboratories will take at least a week to process samples and report the results back to the grower. In many cases, laboratories do not interpret results or recommend how to remedy the situation.
Leaf analysis in cucumbers is based on a sample of the youngest fully mature leaf (with petiole) at the early flowering stage. Table 3.1 lists the standards used to interpret the leaf analysis report.
Table 3.1: Leaf analysis standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage). Source: Ankerman & Large, 1992.
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Nitrogen |
% |
1.8 |
1.8–2.5 |
3.5–5.5 |
5.5–7 |
>7 |
|
Phosphorus |
% |
0.20 |
0.20–0.3 |
0.3–0.7 |
0.7–1.0 |
>1.0 |
|
Potassium |
% |
2.0 |
2.0–3.0 |
3.0–4.0 |
4.0–5.0 |
>5.0 |
|
Calcium |
% |
1.0 |
1.0–2.0 |
2.0–3.0 |
|
|
|
Magnesium |
% |
0.15 |
0.15–0.6 |
0.6–1.5 |
1.5–2.5 |
>2.5 |
|
Sulfur |
% |
|
0.3 |
0.3–1.0 |
|
|
|
Sodium |
% |
|
|
0–0.35 |
>0.35 |
|
|
Chloride |
% |
|
|
0–1.5 |
1.5–2.0 |
>2.0 |
|
Copper * |
mg/kg |
3 |
3–10 |
10–20 |
20–30 |
>30 |
|
Zinc * |
mg/kg |
15 |
15–30 |
30–70 |
100–300 |
>300 |
|
Manganese * |
mg/kg |
15 |
15–50 |
50–200 |
200–500 |
>500 |
|
Iron |
mg/kg |
‡ |
50 |
50–200 |
|
|
|
Boron |
mg/kg |
20 |
20–30 |
30–70 |
70–100 |
>100 |
|
Molybdenum |
mg/kg |
0.2 |
0.2–0.5 |
0.5–2.0 |
|
|
*Values for copper, zinc and manganese in leaves sprayed with fungicides or nutrient sprays containing trace elements cannot give a reliable guide to nutritional status.
‡ Leaf analysis is not a reliable guide to iron deficiency because of surface contamination with soil, immobility of iron within the plant, or the presence of physiologically inactive iron within tissues.
Table 3.2: Leaf analysis standards for greenhouse cucumbers (in dry matter of 3rd-5th leaves from the top, Source: C. de Kreif, et al., 1992.
|
Nutrient |
Unit |
Deficient |
Normal |
Excessive |
|---|---|---|---|---|
|
Nitrogen |
% |
1.8 |
4.2-5.6 |
|
|
Phosphorus |
% |
0.47 |
0.6–0.9 |
|
|
Potassium |
% |
2.35 |
3.2–4.5 |
|
|
Calcium |
% |
1.2 |
2.2–2.4 |
|
|
Magnesium |
% |
0.37 |
0.4–0.7 |
|
|
Sulfur |
% |
|
0.3–0.6 |
|
|
Copper |
mg/kg |
5 |
5–17 |
|
|
Zinc |
mg/kg |
26 |
50–140 |
>300 |
|
Manganese |
mg/kg |
15 |
55–300 |
>550 |
|
Iron |
mg/kg |
|
85–300 |
|
|
Boron |
mg/kg |
43 |
50–76 |
>108 |
|
Molybdenum |
mg/kg |
0.29 |
1.0–2.0 |
|

3.2 Nitrogen (N)
Most plants need nitrogen in large amounts. It is generally considered to drive plant growth. Nitrogen plays an essential role in the composition in all proteins in all plants. Since it is a major structural and functional factor for every plant, crop yields are highly dependent on N availability to the plant.
Nitrogen is required in the production of chlorophyll (the green pigment in leaves), which is responsible for converting sunlight to usable plant energy. Therefore, shortage of nitrogen reduces the plant's capacity to trap energy through photosynthesis. It is important that when reaching the flowering stage the plant will be well developed vegetatively; or it will have a low yielding potential.
3.2.1 Nitrogen application form: nitrate, ammonium, urea and ammonia
The form in which N is supplied is of major importance due to the crop's sensitivity to the status of related nutrients, as demonstrated in many studies, four of which are cited hereby:
- In hydroponics culture, NO3- nutrition results in greater plant growth than with either ammonium-nitrate or urea nutrition.
- Uptake of K is greatly inhibited in cucumber seedlings (cv. Saticoy) fed by nutrient solution containing NH4+ + NO3- (2:1 ratio), compared to NO3- only (Figure 3.1). Moreover, this study also established that the ammonium ion doubles the chloride influx into roots, and this inhibits growth of the seedlings. As shown in paragraph 2.2.10 (page 25), the Cl- anion is specifically toxic to cucumber seedlings.
- The ratio NO3- / NH4+ influences the concentration of K in the sap of cucumber seedlings. The higher the ratio, the higher is the concentration of K in the xylem sap (Figure 3.2). Another study showed that chloride content in the plant sap was markedly increased with increased ammonium.
- When nitrogen is supplied as ammonia (NH3) both shoot and root biomasses, yield and K content decrease, possibly because ammonia causes morphological changes in the roots that interfere with their ability to absorb nutrients. Moreover, ammonia also inhibits roots respiration, resulting in net efflux of K.
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Nitrogen |
% |
1.8 |
1.8–2.5 |
2.5–4.5 |
4.5–6 |
>6 |
Figure 3.1: The effect of nitrogen form on K uptake (Martinez and Cerda, 1989).
Figure 3.2: The effect of NO3-/NH4+ ratio in the soilless nutrient solution on the concentration of K in the sap of cucumber (cv. Hyclos) seedlings (Zornoza and Carpena, 1992).
3.2.2 Nitrogen deficiency
Failure to supply ample available nitrogen causes bleaching of the older leaves, (Figure 3.3), impaired plant development, delayed growth, thin stems (Figure 3.4) and distorted and discolored fruit (Figure 3.5). In severe cases, the whole plant collapses. Nitrogen deficiency may render the cucumber plant very sensitive to salinity.
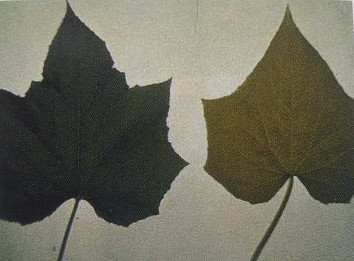
Figure 3.3: Typical nitrogen deficiency symptoms in cucumber leaves. On the left: Healthy; on the right: Deficient.

Figure 3.4: Left: Completely stunted and pale plant that has not received any N after Germination. Center: A healthy plant. Right: A plant that received less N than the center one. Older leaves turn pale green to yellow; this discoloration spreads to younger leaves up the plant.

Figure 3.5: Nitrogen deficient cucumber fruit is misshapen and chlorotic.
Both vegetative growth and fruit production are severely restricted when nitrogen supply is inadequate. Plants appear pale and spindly. New leaves are small but remain green, whereas the oldest leaves turn yellow and die. If the problem is not corrected, the yellowing spreads up the shoot to younger leaves. Yield is reduced and fruit are pale, short and thick.
Treatment
In soil-grown crops: side-dress with 20–50 kg/ha of N, or apply fortnightly foliar sprays of 2% urea at high volume. To prevent possible salt burn to leaves when applying any foliar spray, spray late in the afternoon or during cloudy weather.
For crops grown in soilless media, use a nutrient solution containing 150–200 ppm N.
3.2.3 Excessive nitrogen
Plants given slightly too much nitrogen show excessive growth and a softer plant; leaves will be darker green and sometimes thickened and brittle.
Plants given highly too much nitrogen are generally stunted and have strong, thick stems, short internodes, a mass of tendrils, short side shoots, fewer flowers, and small fruit. The middle and older leaves cup downwards and wilt easily in warm conditions. Leaf scorching is common. Transparent spots occur between the veins or at the leaf edges, which eventually turn yellow and then brown.

Figure 3.6: Symptoms of excessive nitrogen include wilting and downward cupping of the older leaves, followed by yellow and brown burnt areas on lower leaves.
Treatment
Leach the soil or growing medium with fresh water to remove the excess fertilizer.
Adjust the fertilizer program to ensure that rates do not exceed crop needs.
3.3 Phosphorus (P)
Phosphorous is essential for the normal development of the roots and reproductive organs (flowers, fruit, seeds). Phosphorus promotes strong early plant growth and development of a strong root system. Highly available phosphorous is needed for the establishment of the seeded or transplanted cucumbers. Phosphorus is required for cellular division and formation of molecules taking part in energy transformation (ADP & ATP).
Plants require phosphorus at all stages of growth, but demand is greatest during crop establishment and early plant growth. If phosphorus becomes limiting, it is translocated from older to younger tissues, such as the leaves, roots and growing points. In a crop such as cucumbers, which has a continuous production of new vegetative and fruiting tissues, a regular supply of phosphorus (and other elements) is needed to ensure that the plant can sustain quality fruit production over a prolonged period.
For soil-grown crops, P availability is usually optimal when the soil pH is between 6.0 and 6.5.
In acid soils (pH 6.0), the P is associated (“tied up” or “fixed”) with iron and aluminum compounds that are slowly available to most plants.
In soil where pH is greater than 6.5, the P is primarily associated with calcium and magnesium. The higher the pH calcium- and magnesium- phosphate compounds are less available to plants.
Table 3.4: Leaf analysis phosphorus standards for field-grown cucumbers (in dry matter of
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Phosphorus |
% |
0.20 |
0.20–0.3 |
0.3–0.7 |
0.7–1.0 |
>1.0 |
3.3.1 Phosphorus deficiency
When soils are low in phosphorus, they must be regularly supplemented with fertilizer. To ensure uninterrupted phosphorus supply, a P- fertilizer must be applied to the soil before planting. A soil test is therefore essential to establish the soil’s phosphorus status and to estimate how much (if any) phosphorus fertilizer is required.
Phosphorus-deficient plants have weak roots, are stunted, and produce small, dark, dull, gray-green leaves (Figure 3.7). The oldest leaf, at the base of the shoot, turns bright yellow (Figure 3.8).
However, unlike nitrogen deficiency, the leaf directly above this leaf remains dark green (Figure 3.9). Brown patches appear between the veins on mature leaves. These become scorched and spread until the leaf dies prematurely. Fruit set is reduced, so production is impaired until the deficiency is corrected.

Figure 3.7: Left: A phosphorous deficient plant is stunted and has small, dark green, dull leaves. Right: A healthy plant.

Figure 3.8: An older leaf of a P deficient plant shows brown, scorched spots between the veins.

Figure 3.9: A P-deficient plant. The oldest leaf of this stunted plant is bright yellow, but the leaf above it remains dark green.
Treatment
A soluble phosphorus source such as mono potassium phosphate (e.g. Haifa MKP) can be supplied through fertigation(Nutrigation™) or by foliar spray, to revive established crops with deficiency symptoms. These treatments should be repeated for the rest of the crop life. Soil-grown crops will not respond immediately to these treatments.
For crops grown in soilless media, use a nutrient solution containing 25–50 ppm P.
3.3.2 Phosphorus toxicity
Phosphorus toxicity is uncommon in soil-grown crops but can occur in hydroponic crops.

Figure 3.10: Various P-toxicity symptoms on a cucumber plant.
3.4 Potassium (K)
3.4.1 The role of potassium in cucumber nutrition
Potassium is a major nutrient that plays a key role in many physiological processes in all plants among which are:
- Activation at least 60 different enzymes involved in plant growth and metabolism.
- Regulation of the water balance of the plant through the roots osmotic gradient and the functioning of stomata guard cells.
- Involvement of the activation of several enzymes and control of ATP formation as a part of photosynthesis.
- Enhancing the translocation of nutrients through the xylem system and of organic compounds, mainly carbohydrates, in the phloem system from source to sink.
- Involvement in any major step of protein synthesis.
- Reduction of plant susceptibility to plant disease, and a-biotic stresses.
- Counteracts salinity.
Adequate potassium levels enhance the production and floem transportation of carbohydrates within the plant. Potassium also play an important role in enhancing plant resistance to low temperatures, salinity, drought and diseases.
Ample amounts of potassium should therefore be supplied to the crop to ensure abundant K levels in all major organs.
Severe potassium deficiency will retard the transportation of sugars within the plant, leading to starch accumulation in the lower leaves.
Cucumbers are unique among most crops in their high potassium requirements. In fact, the cucumber plant is one of the only crops requiring more potassium than nitrogen (Figure 3.11).
Moreover, increase in cultivation intensity requires a higher K/N ratio (Table 3.5).
Figure 3.11: N-P-K uptake rates by cucumber crops at various growing conditions and yields.
Table 3.5: Indicative N-P-K ratios at various growing conditions (La Malfa, 1992)
|
|
Open Field |
Greenhouse |
|
Expected yield (Ton/ha) |
15 – 30 |
120 - 300 |
|
Typical N:P:K ratio |
1:0.5:1.5 |
1:0.5:2 |
High concentrations of K are found in various plant parts, and serve as a method to assess crop nutritional status. On a whole organ basis, potassium is generally equally distributed between roots and shoots.
Table 3.6: Leaf analysis potassium standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Potassium (K) |
% |
2.0 |
2.0–3.0 |
3.0–4.0 |
4.0–5.0 |
>5.0 |
Normal levels in petiole sap are 3,500 – 5,000 ppm K.
The accumulation of K in stems, petioles and leaves correlates with the growth curve of these organs; this suggests a mechanism that balances K uptake and release. K accumulates rapidly in each leaf during its early developmental stage (Figure 3.12). The overall demand of the plant leaves creates therefore a sigmoid ascending curve.
3.4.2 Potassium enhances cucumber yield
a) Soilless on peat bags, potassium applied by fertigation
It has been shown clearly the positive effect of potassium fertilization on cucumber yields. When grown on peat bags, a potassium level of 50 mg/l in liquid feed by fertigation produces a mean yield of 9.98 kg/plant of cucumbers.
Yield was increased by 91% to 19.1 kg/plant when the potassium level was raised from 50 to 250 mg/l (Figure 3.13).
Figure 3.13: Effect of fertigated K on the yield of soilless cucumber (cv. Femdam), (Adams et al., 1992).
Another study has shown a clear positive response of cucumber productivity to potassic nutrition of the plants. Total number of fruit units was increased by 15% as a result of a slight increase of additional 100 ppm in potassic fertilization.
b) Open field, potassium applied by side dressing
A very similar response was also obtained in open field conditions. The research was conducted during two consecutive years and showed remarkable positive reaction of cucumber yield to intensive potassic fertilization (Figure 3.14).
Figure 3.14: Effect of side-dressed K on the yield of field-grown cucumbers (cv. Poinsett), (Forbes and White, 1986).
3.4.3 Potassium enhances cucumber disease resistance
Potassium has a well-known effect of both enhancing plant resistance to pathogens and of reducing the impact of the infection. The following results have been reported for cucumbers in this context:
- Potassium lessens the effect of Angular Leaf Spot caused by Pseudomonas lachrymans.
- Fruit gray mold caused by Botrytis cinerea is reduced by 27% - 33% by the application of supplemental K to parthenocarpic cucumbers grown in tunnels.
- Foliar sprays of potassium salts solutions have been shown to control Powdery Mildew caused by Sphaerotheca fuliigiena (Figure 3.15). The researchers postulated that the foliar spray produces systemically induced resistance.

Figure 3.15 A: Up: A cucumber leaf heavily infected by Powdery Mildew.

Figure 3.15 B: Leaves from adjacent plant treated with Multi-K™.
The results shown in Figure 3.16 demonstrate the remarkable results of reducing the damage to cucumbers from this fungus by spraying potassium nitrate (e.g. Multi-K™).
Figure 3.16: The effect of foliar sprays of Multi-K™ on powdery mildew in cucumbers (cv. Delila), (Reuveni et al., 1996)
A remarkably increased resistance of cucumber plants (cv. Corona) to infection by Pythium ultimum was brought about by enhanced potassic nutrition. Shoots dry weight was increased by 20%, roots dry weight – by 28%, and number of class 1 fruits – by 71%.
3.4.4 Potassium deficiency symptoms
Potassium deficiency will take place when the concentration of K falls to below 3,000 ppm in the petiole sap or below 3.5% in the dry weight of the leaf laminae. Such deficiency may develop when cucumbers are grown in soil and sufficient K is not available to the plant during the growth period, as required by the plant.
Deficiencies will develop very quickly in soilless culture if adequate rate of potassium is not regularly maintained in the irrigation solution.
- Symptoms of potassium deficiency first appear on older leaves. Typically,chlorosis first appears at the leaf margins, then, the interveinal area is affected. The symptoms progress from the base towards the apex of the plant. Yellowing and scorching of the older leaves begins at the edges and eventually spreads between the main veins towards the center of the leaf (Figure 3.17).
- Leaf symptoms are accompanied by abnormal fruit development, often with a brown or spotted appearance.
- The plant is stunted with short internodes and small leaves.

Figure 3.17: The potassium deficient plant has yellow scorched older leaves, which turn dry and papery.


Figure 3.18: Typical potassium deficiency symptoms as shown on the leaves.
Potassium deficiency influences plant water regulation by affecting cell turgor and the opening and closing of stomata, hence, deficient crops are prone to wilting.
As potassium is mobile in the plant, it moves to the younger leaves when supplies are short.
Although the growth of deficient plants may not be seriously impaired, the yield and quality of fruit are often greatly reduced. Fruit may not expand fully at the stem-end, although they will swell at the tip-end, a symptom that is also caused by water stress (Figure 3.19).

Figure 3.19: Fruit fails to expand at the stem end.
Treatment
- In medium or heavy soils, where potassium movement is rather slow, potassium nitrate (e.g. prilled Multi-K™ should be incorporated in the soil before planting. An early soil test can be used to determine the rate needed.
- In sandy soils, where quick response to potassium fertilizer is expected, an application of side-dressed soluble Multi-K™, may result in fast correction of the deficiency.
- Nutrigation™ (fertigation) feeding with soluble Multi-K™ can also be used to treat a deficient crop.
- Foliar sprays with soluble Multi-K™ or Poly-Feed™ is effective and can cure the K deficiency within a short period of time (see pages 90-91).
- For crops grown in soilless media, use a nutrient solution containing 150–200 ppm K.
Do not over apply potassium fertilizer, especially in light soil or in soilless systems, as excessive K causes N deficiency in the plants and may affect the uptake of other cations such as Mg and Ca.
3.4.5 Interrelationships between potassium and other nutrients
- An over-optimal level of either potassium or nitrogen may induce or accentuate a phosphorous deficiency.
- An over-optimal potassium level may induce Mg deficiency and decrease yields in cucumber grown in peat.
- A K/Ca ratio of 1.33 was found optimal for nutrient solution, in greenhouse- grown cucumbers is sand culture. Under such conditions highest yields and highest Ca uptake were obtained.
- Potassium deficiency can induce or accelerate iron deficiency, and enhance nitrogen accumulation.
3.5 Calcium (Ca)
Calcium takes a key role in the structure and functioning of cell membranes and the strength of cell walls. Calcium also reduces plant susceptibility to diseases. Most calcium-related disorders of crops are caused by unfavorable growing conditions and not by inadequate supply of calcium to the roots. Rapidly growing crops in hot windy conditions are most at risk. Deficiencies can also develop when cucumbers grow quickly under continuously humid conditions, as in a greenhouse. Other contributing factors are waterlogging, soil salinity, high potassium or ammonium supply, and root diseases.
Calcium moves in the plant’s transpiration stream and is deposited mainly in the older leaves.
Deficiencies are found in the youngest leaves and growing points, which have low rates of transpiration. Emerging leaves appear scorched and distorted and may cup downwards because the leaf margins have failed to expand fully (Figure 3.20). Mature and older leaves are generally unaffected. With a severe deficiency, flowers can abort, and the growing point may die. Fruits from calcium-deficient plants are smaller and tasteless, and may fail to develop normally at the blossom end.

Figure 3.20: Youngest leaves of calcium deficient plants cup downwards and their edges become scorched.

Figure 3.21: Calcium deficient cucumber leaves.
Table 3.7: Leaf analysis calcium standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Calcium |
% |
1.0 |
1.0–2.5 |
2.5–5.0 |
|
|
Treatment of calcium deficiency
Applying lime to acidic soils and reducing the use of ammonium-based fertilizers is recommended to avoid insufficient calcium availability in the soil.
Existing injury from calcium deficiency can be reduced by regular foliar sprays of fully soluble calcium nitrate, (15.5-0-0+26.5 CaO), e.g. Haifa Cal, at 800g / 100L).
For crops grown in soilless media, use a nutrient solution containing fully soluble calcium nitrate, (15.5-0-0+26.5 CaO), e.g.Haifa Cal at a rate of 150–200 ppm Ca. Keep the conductivity of water in the growing medium below 2 dS/m as the incidence of deficiency increases with increasing conductivity.
Excessive Ca may be problematic when growing cucumbers on calcareous soils. High Ca in the soil usually causes high pH which then precipitates many of the micronutrient so they become unavailable to the plant, it may also interfere with Mg absorption.
3.6 Magnesium (Mg)
Magnesium is a center constituent of the chlorophyll molecule, so its key role in the photosynthesis process is crystal-clear. Magnesium deficient soils may be found in coarse-textured soils in humid regions, and especially on light, sandy, acidic soils in higher-rainfall areas. Magnesium deficiency can be also induced by heavy rates of potassium, ammonium or calcium (heavy liming) fertilizers. Symptoms are more likely to show during cold weather or on heavy wet soils, when roots are less active.
Magnesium deficiency causes yellowing of older leaves. (See Figures 3.22 & 3.23.) The symptom begins between the major veins, which retain a narrow green border. A light tan burn will develop in the yellow regions if the deficiency is severe.
Fruit yields are reduced.

Figure 3.22: Yellowing between the major veins of older leaves (left) turns to a light tan papery burn (right). Younger leaves (top) are less affected.

Figure 3.23: Yellowing and light tan burn on older leaves of magnesium deficient plant.
Table 3.8: Leaf analysis magnesium standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Magnesium |
% |
0.15 |
0.15–0.3 |
0.3–1.5 |
1.5–2.5 |
>2.5 |
Treatment
- Incorporate into deficient soils before planting any of the following magnesium-rich minerals:
– Magnesite (MgCO3 - 28.8 % Mg, or 47.8 % MgO), at 300 kg/ha.
– Dolomite (CaMg(CO3)2 21.7 % Ca, or 30.4% CaO, and 3.2 % Mg, or 21.96% MgO), at 800 kg/ha. - A deficiency in a current crop can be corrected by fortnightly foliar sprays of fully soluble magnesium nitrate (11-0-0+16 MgO), e.g. Magnisal™ at 2 kg / 100 L) at volume of 500–1,000 L/ha.
- For crops grown in soilless media, use Magnisal™ in a nutrient solution containing 30 ppm Mg.
3.7 Sulfur (S)
The main function of sulfur in the plant is as a core constituent of the essential amino acids cystin, cystein and methionine, as well as taking part in the composition and functioning of many coenzymes.
The vitamins Thiamine and Biotin use sulfur, so this element plays a key role in plant metabolism. Hence, sulfur deficiency will result in deficient proteins, in a way that resembles nitrogen deficiency. But, as sulfur has low mobility in the plant its deficiency symptoms will show firstly on the newly formed, young leaves, as negated to nitrogen deficiency.
Sulfur is generally present at adequate amounts in soil-grown cucumbers that receive sulfate salts of potassium or magnesium.
When it occurs sulfur deficiency shows as a general yellowing of the entire foliage, especially- on the new growth. This can be differentiated from similar symptoms produced by iron and manganese deficiency, as with sulfur deficiency the veins of the leaves do not remain green, but the leaf blade takes on a dull uniform yellow coloration.
Sulfur levels in hydroponic solutions may be in the range of 20-200 ppm, because plant requirements of this element are pretty flexible between quite a wide range.
Table 3.9: Leaf analysis sulfur standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Sulfur |
% |
|
0.3 |
0.3–2.0 |
2.0-4.0 |
|
Excessive sulfur in the soil (seldom) may cause premature dropping of leaves.
3.8 Manganese (Mn)
The function of manganese in the plant is closely associated with the function of iron, copper and zinc as enzyme catalysts. Manganese is needed for photosynthesis, respiration, nitrate assimilation and the production of the plant hormone auxin.
Without manganese, hydrogen peroxide accumulates in the cells and damages them. Like iron, manganese is immobile within the plant, accumulating mostly in the lower leaves.
Deficiencies are more likely in calcareous or alkaline soils, or over-limed soils; availability is high in acidic soils.
The veins of middle to upper leaves of manganese-deficient plants remain green while the rest of the leaf becomes a uniform pale green to yellow. (See Figure 3.24.)
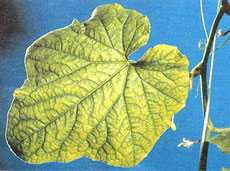
Figure 3.24: Typical color contrast between the veins and the rest of the leaf blade of middle to upper sulfur deficient cucumber leaves
Table 3.10: Leaf analysis manganese standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Manganese * |
mg/kg |
15 |
15–60 |
60–400 |
400–500 |
>500 |
Treatment
- Spray the foliage with manganese sulfate (100 g/100 L).
- For crops grown in soilless media, use a nutrient solution containing 0.3 ppm Mn.
Manganese toxicity
- Found under strongly acid conditions.
- Numerous small reddish-brown spots appear between the veins of the oldest leaves and on leaf petioles of plants with excess manganese (Figure 3.25). With time, the tissue around each spot becomes chlorotic, and the older leaves turn pale and age prematurely.
- Manganese toxicity may show by reduction in growth.
- Cucumbers are not particularly sensitive to excess manganese, and tissue concentrations may be relatively high before toxicity symptoms show.
- Manganese toxicity can induce iron deficiency.

Figure 3.25: Small reddish-brown spots appear between the veins of the oldest leaves of Mn-excessive cucumber leaves.
Treatment
Manganese toxicity in soil-grown crops is associated with acidic conditions and waterlogging.
Correction therefore involves liming and improving drainage and irrigation scheduling.
3.9 Iron (Fe)
Iron is needed to produce chlorophyll and to activate several enzymes, especially those involved in photosynthesis and respiration. Iron is immobile in the plant.
Deficiencies of iron are more likely in alkaline or calcareous soils, and can be induced by over-liming, poor drainage, or high concentrations of metallic ions in the soil or nutrient solution. Iron availability decreases at pH above 7. Manganese toxicity can induce an iron deficiency.
Iron deficiency causes a uniform pale green chlorosis of the newest cucumber leaves; all other leaves remain dark green. Initially, the veins remain green, which gives a net-like pattern. If the deficiency is severe, the minor veins also fade, and the leaves may eventually burn, especially if exposed to strong sunlight.

Figure 3.26: Youngest leaves (left and center) are pale green to yellow with
green veins. In severe cases (center) of iron deficiency, the minor veins also
fade, and affected leaves appear light yellow to white. On the right, a healthy leaf.

Figure 3.27: Pale green symptoms first appear on young leaves.
Treatment
The best long-term action is to correct soil chemical and physical problems. Good drainage and soil aeration favor iron availability.
Foliar sprays of iron sulfate (150 g/100 L) can be used to treat symptoms, but symptoms will return if the sprays are discontinued.
For crops grown in soilless media, use a nutrient solution containing 2–3 ppm Fe. Iron chelates are generally less likely to precipitate under alkaline conditions and are normally preferred in hydroponic solutions.
Table 3.11: Leaf iron analysis standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Iron |
mg/kg |
|
50 |
50–300 |
|
|
Leaf analysis is not a reliable guide to iron deficiency because of surface contamination with soil, immobility of iron within the plant, or the presence of physiologically inactive iron within tissues.
Iron toxicity will be revealed by bronzing of leaves with tiny brown spots.
3.10 Boron (B)
Boron is important in the regulation of developing cells and in pollination. Seed set and fruit development are affected by deficiency. Plants are rather exacting in their requirements for boron, and the margin between deficiency and excess is particularly narrow. For example, in mini cucumbers, a leaf boron concentration of 30–70 μg/g is desirable, but deficiency occurs at less than 20 μg/g and toxicity- at more than 100 μg/g.
Boron is not readily moved from old to new tissues; hence continuous uptake by roots is needed for normal plant growth.
Boron deficiency causes both leaf and fruit symptoms. The main leaf symptoms are a distortion of newer leaves (in severe cases the growing point dies) and the appearance of a broad yellow border at the margins of the oldest leaves.
Young fruit will die or abort at high rates (Figure 3.29).

Figure 3.28: Boron-deficient cucumber leaves.

Figure 3.29: Aborted fruit (top); twisting and scarring (center and bottom).
The symptoms of boron deficiency on mature fruit are distinctive and include stunted development and mottled yellow longitudinal streaks, which develop into corky markings (scurfing) along the skin.
These symptoms are often most severe near the blossom end of the fruit. Similar symptoms can occur on fruit grown in greenhouses with inadequate winter heating. Developing and mature fruit can taper and curve at the blossom end. The proportion of pith to seed is often higher in boron-deficient fruit.
Not to be confused with symptoms of fruit scurfing and severe twisting with damage by western flower thrips.
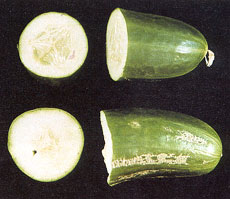
Figure 3.30: Deficient fruit (bottom) have a high proportion of pith to seed and corky markings on the skin.

Figure 3.31: Longitudinal, mottled yellow streaks (left and center) develop into corky markings on the skin (right).
Treatment
One needs to be very careful when treating a boron-deficient crop, as excessive boron can be toxic, and boron is highly toxic to most plants at quite low rates. Trial foliar sprays are advisable. Take care with soil treatments to ensure an even spread, and be aware that
An application of 10 kg borax per hectare to deficient soil before planting will prevent boron deficiency. Foliar sprays of borax (100 g/100 L) may also be used.
For crops grown in soilless media, use a nutrient solution containing 0.3 ppm B.
Table 3.12: Leaf boron analysis standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Boron |
mg/kg |
20 |
20–30 |
30–70 |
70–100 |
>100 |
Boron toxicity
Cucumber plants are very sensitive to high boron levels.
Boron toxicity is indicated by yellowing between the veins of older leaves. This is followed quickly by the development of small, brown necrotic spots, which eventually join to form large areas of dead tissue. At the same time, the newer leaves become chlorotic and are distorted because of damage in the bud. Few female flowers may develop.

Figure 3.32: Boron toxicity in older leaves, including yellowing between the veins (left) followed by small brown necrotic spots (center) and large areas of dead tissue (right).
Treatment
Boron toxicities are harder to correct than deficiencies.
Problems in crops are usually caused by careless application of boron. When this happens, the soil can be flushed with fresh water to remove the excess.
3.11 Zinc (Zn)
Zinc contributes to the formation of chlorophyll and the production of the auxins plant hormones.
It is an integral part of several plant enzymes. Zinc deficiency appears as a distortion and interveinal chlorosis (yellowing) of older plant leaves, and retards plant development as a consequence of low auxin levels in the tissue.
In soils zinc becomes less available as the soil pH rises and in the presence of calcium carbonate. A heavy application of phosphorus can induce zinc deficiency due to the precipitation of the zinc in the form of zinc-phosphates. Uptake of zinc is hindered by over-optimal copper, iron, manganese, magnesium, and calcium. The normal zinc content of soils usually falls in the range of 10-300 ppm Zn.
Hydroponically grown cucumbers develop zinc deficiency when plants have too low zinc in the nutrient solution. Normal zinc levels in hydroponic solutions runs at 0.1-0.5 ppm.
The symptoms of deficiency are not well defined, but usually a slight interveinal mottle develops on the lower leaves, this symptoms spreads up the plant. The upper internodes remain short. Small leaf size most characterizes zinc deficiency; in severe cases, short internodes cause the top of the plant to grow bushy. Overall growth is restricted and the leaves become yellow-green to yellow except for the veins, which remain dark green and well defined.
Symptoms of deficiency appear when the concentration drops below 15-20 ppm Zn.
Treatment
Spraying with zinc sulfate (5 g/L) easily corrects a zinc deficiency.
Table 3.13: Leaf zinc analysis standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Zinc * |
mg/kg |
15 |
15–20 |
20–100 |
100–300 |
>300 |
*Values for zinc in leaves sprayed with fungicides or nutrient sprays containing trace elements cannot give a reliable guide to nutritional status.
Zinc toxicity
Zinc toxicity can be caused by contamination of the water used in soilless culture. Contact of corrosive nutrient solutions with galvanized pipes or fittings has been known to lead to zinc toxicity in sensitive seedlings. Galvanized greenhouse frames and wires are other possible sources of excess zinc.
Toxicity occurs in soils contaminated by their proximity to zinc smelters and mines.
Toxicity can be expected when the zinc concentration exceeds 150-180 ppm (old leaves) or 900 ppm (tops of plants).
In the case of zinc toxicity, the entire veinal network, initially dark green, becomes somewhat blackened. The blackish appearance of the main veins helps distinguish zinc toxicity from manganese deficiency in which the veins remain green. In severe cases of zinc toxicity the symptoms resemble those of iron deficiency.
Zinc toxicity causes a pale green chlorosis of newer leaves. If toxicity is severe, pinhead-sized light-brown spots may appear between the veins. Older leaves may wilt and appear dull. All leaves are a lighter green than is normal.
Other zinc toxicity symptoms include sever stunting, reddening; poor germination; older leaves wilt; entire leaf is affected by chlorosis, edges and main vein often retain more color.

Figure 3.33: Zinc toxicity in cucumber leaves, pale-green chlorosis. Young leaves become yellow.
Treatment
Remove eroding galvanized metal parts. Apply lime or phosphate.
3.12 Molybdenum (Mo)
Molybdenum is involved in many enzymes and is closely linked with nitrogen metabolism as it is an important component of nitrate-reductase and nitrogenase enzymes.
Molybdenum is present in soil as an anion, in contrast to most other micronutrients, which are present as cations. The availability of molybdenum increases as the pH rises and therefore a deficiency of this element is more likely to occur in acid (and sandy) soils, in which case liming might be helpful.
Plants need tiny amounts of molybdenum, an average 0.2 ppm Mo available in soils is adequate.
Molybdenum deficiencies are rare, but have been observed in plants growing in peat.
Initially, the green of the leaves fades, particularly between the veins. Later, leaves can turn yellow and die. In some cases, parts of mature leaves remain green at first, giving rise to a blotchy appearance. Symptoms start first in lower leaves and spread upwards, the younger ones remaining green. Growth might appear normal but flowers stay small. Severe deficiency cases in peat can significantly reduce yield (up to 84%), but raising the pH (up to 6.7) through liming restores yield to near normal.
Treatment
As a preventative measure on peat, apply sodium molybdate at 5 g/m³. Treat a deficiency either by applying sodium molybdate to the soil at 150 mg/m² or by spraying with a solution of sodium molybdate in water at 1 g/L.
Toxicity
Plants can take up high levels of molybdenum without harmful effects on growth, Intense yellow or purple color in leaves may be rarely observed.
There might be concern for human health with high molybdenum levels in the produce.
Table 3.14: Leaf analysis molybdenum standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Molybdenum |
mg/kg |
0.2 |
0.2–0.5 |
0.5–2.0 |
|
|
3.13 Copper (Cu)
Several enzymes with diverse properties and functions depend on copper, including those involved in photosynthesis and respiration. Although copper is mobile in plants well supplied with the element, it is much less mobile in deficient plants. Therefore copper concentration in young developing tissue is likely related to plant status. However, soil analysis is a more useful guide to copper deficiency than tissue analysis.
Copper deficiency is unusual, partly because the widespread use of copper in plumbing and in fungicides ensures an adequate supply in most cases. Occasionally it becomes a problem with crops in peat media or in all plastic hydroponic systems when no copper is added to the nutrient solution. High soil pH reduces available copper, but this effect is much smaller than for manganese, iron, and boron.
Copper deficiency symptoms
Restricted growth, short internodes and small leaves. Initially, interveinal chlorotic blotches appear on mature leaves, but later symptoms spread upwards on the plant. The leaves eventually turn dull green or bronze, their edges turn down, and the plant remains dwarfed.
Furthermore, bud and flower development at the top of the plant decreases.
Copper deficiency can dramatically reduce yield (by 20-90%). The few fruits that are produced develop poorly with small, sunken brown areas scattered over their yellow-green skin.
Treatment
To prevent copper deficiency in peat media, where it is most common, add copper sulfate at 10 g/m³, as a precaution. Generally, nutrient solutions should contain 0.03 ppm Cu. For quick results, spray plants with a solution of copper sulfate using up to 1 g/L, plus calcium hydroxide (approx. 0.5%) for pH adjustment.
Table 3.15: Leaf analysis copper standards for field-grown cucumbers (in dry matter of youngest fully mature leaf with petiole taken at early flowering stage).
|
Nutrient |
Unit |
Deficient |
Low |
Normal |
High |
Excessive |
|
Copper |
mg/kg |
3 |
3–7 |
8–20 |
20–30 |
>30 |
Values for copper in leaves sprayed with fungicides or nutrient sprays containing trace elements cannot give a reliable guide to nutritional status.
Toxicity
Copper toxicity, although rare, can appear as an induced iron chlorosis, where the soil is contaminated with copper either from industrial sources or after repeated spays of copper-containing fungicides. Toxic effects persist, and the only partial solution is heavy liming. In hydroponic systems, extensive use of copper plumbing can produce copper contamination.
Need more information about growing cucumbers? You can always return to the cucumber fertilizer & cucumber crop guide table of contents or the cucumber growth stages
Related Articles:
NPK Fertilizers - Water Soluble Fertilizers
Plant Fertilizer & Plant Nutrition



